Abstract
Background
The utility of high-dose cytarabine during induction chemotherapy for adult acute myeloid leukemia (AML) remains contentious. Intermediate-dose cytarabine may maintain a high first-cycle complete remission (CR) rate, whilst reducing treatment-related toxicity.
Aim
We examined the outcomes of adult AML patients treated with idarubicin (12 mg/m2 days 1-3) in combination with high-dose (3 g/m2; HDAC3) or intermediate-dose cytarabine (1.5 g/m2; IDAC3) given twice daily on days 1, 3, 5, and 7.
Methods
Consecutive AML patients aged ≤60 years at 2 major tertiary referral centers, the Alfred and Austin Hospitals, were treated with sequential protocols of HDAC3 (n=52; 2010-2014) and then IDAC3 (n=58; 2014-2017). Planned consolidation chemotherapy consisted of up to 2 cycles of idarubicin 9 mg/m2 days 1-2, cytarabine 100 mg/m2 days 1-5, and etoposide 75 mg/m2 days 1-5 (IcE). Favorable-risk karyotype was excluded from this analysis. We assessed treatment responses (2017 European LeukaemiaNet recommendations), 30 and 60-day mortality, ability to deliver consolidation therapy (number of cycles and days from induction to first consolidation chemotherapy), rates of allogeneic hematopoietic stem cell transplant (allo-HSCT) in first remission (CR1), rates of total parenteral nutrition (TPN) usage, haematologic toxicities (days to platelet and neutrophil recovery), and relapse-free (RFS) and overall survival (OS) censored for allo-HSCT or loss to follow-up. Statistical analysis was performed using R version 3.3.2.
Results
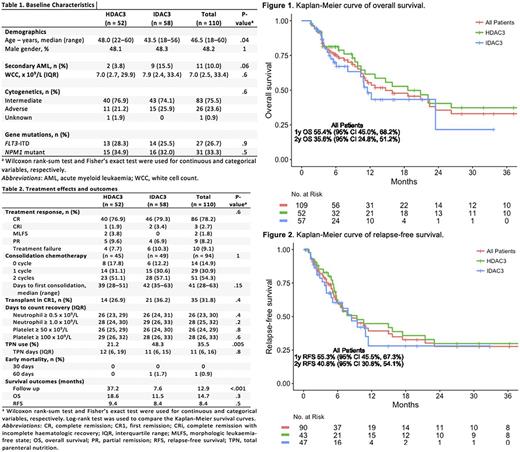
IDAC3-treated patients were younger than those receiving HDAC3 (median 44 vs 48 years) but had a higher frequency of secondary AML (16% vs 4%); (Table 1). Other baseline characteristics were balanced between both groups. No significant differences were observed for any outcome (Table 2), except the use of TPN which is most likely a result of change in practice over time rather than a true reflection of mucositis. Response rates including CR or CR with incomplete haematologic recovery (CRi) were similar: 79% (HDAC3) vs 83% (IDAC3) after 1 cycle of induction. There was no death at 30 days and only 1 death at 60 days. Allo-HSCT in CR1 was similar; among patients with adverse-risk karyotype, allo-HSCT in CR1 was performed for 9/15 (60%) after IDAC3 and 7/11 (64%) after HDAC3. Days to neutrophil and platelet recovery were also similar.
Delivery of consolidation chemotherapy was similar between both groups. After excluding treatment failure and partial remission (PR), 94 patients were eligible for first consolidation chemotherapy (3 patients with PR were considered near CR), of which 14 (15%) did not receive further consolidation therapy with the following reasons: allo-HSCT in CR1 (n=10); prolonged cytopenias (n=3); and maintenance clinical trial (n=1). Twenty-nine patients (31%) received only 1 consolidation therapy due to the following reasons: allo-HSCT in CR1 (n=14); prolonged cytopenias (n=10); non-hematologic toxicities (n=2); relapse (n=2); and unknown (n=1). IcE chemotherapy was administered in all except 2 cases: one received high-dose cytarabine for 1 cycle, and another had reduced idarubicin dose for 1 cycle.
Survivors were followed up for a median of 8 (IDAC3) and 37 months (HDAC3), respectively. Median OS were 11.5 and 18.6 months for IDAC3 and HDAC3 (p=0.3), respectively (Figure 1A), and the corresponding median RFS were 8.4 and 9.4 months (p=0.5), respectively (Figure 1B). Overall, the median OS for adverse-risk karyotype remained significantly inferior compared to intermediate-risk (5.6 vs 20.9 months, p=0.009 by log-rank test); among adverse-risk karyotype, this was 5.1 vs 6.5 months for IDAC3 and HDAC3, respectively. Similarly, patients with FLT3 -ITDpos/ NPM1WT had inferior median OS (n=7, 6.1 months) when compared with FLT3 -ITDneg/ NPM1WT (n=55, 15.9 months), FLT3 -ITDpos/ NPM1MUT (n=14, 20.9 months), and FLT3 -ITDneg/ NPM1MUT (n=16, 40.6 months, p=0.06 by log-rank test).
Conclusion
IDAC3 and HDAC3 were both well tolerated in adult patients with AML with high first-cycle CR/CRi rates observed and low early mortality. No significant differences in hematopoietic recovery or delivery of post-remission therapy was apparent. With the caveat of limited sample size and follow-up duration, survival outcomes appeared equivalent.
Grigg: Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees. Wei: AbbVie, Celgene, Servier: Research Funding; AbbVie, Celgene, Novartis, Amgen, Servier: Membership on an entity's Board of Directors or advisory committees; AbbVie, Celgene, Novartis, Amgen, Servier: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal